How Takeda developed its dengue vaccine after decades of setbacks

After decades of delays, the first vaccine for dengue fever was introduced seven years ago. But that formula, from Sanofi, was soon found to be suitable only for people who had previously been infected with the disease, spurring researchers at Japan's Takeda Pharmaceutical Co. to redouble efforts on an alternative. That work is finally paying off, with their version expected to hit the market early next year.
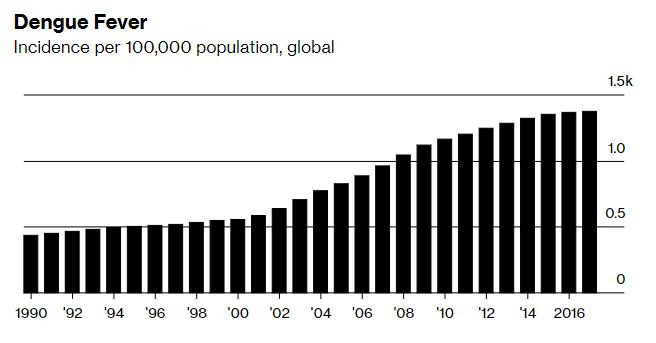
Dengue infections have jumped eightfold over the past two decades, to almost 400 million a year, according to researchers at the University of Oxford. About half the world's population already lives in areas threatened by dengue, and scientists warn that climate change will likely hasten the spread of mosquitoes that carry the virus. Since 2013 the US has seen outbreaks in Florida, Hawaii and Texas. Europe saw local transmission in France and Croatia in 2010, and a 2012 outbreak on Portugal's Madeira island resulted in more than 2,000 cases.
Although most dengue cases are mild, typically causing flulike symptoms, some infected people suffer a sudden onset of fever, headache, rashes and muscle pain so severe that some call the disease "break-bone fever." A half-million dengue patients a year require hospitalization from complications that can result in shock and internal bleeding. About 20,000 die, primarily children.
Indonesia approved Takeda's Qdenga in August, and the company says it will start selling it there early next year. In October a European Union advisory panel said Qdenga can be administered to people as young as 4 years old, a recommendation that could lead to EU consent within a few months. And the company is seeking approvals in about a dozen other countries, including the US.
Qdenga highlights growing successes in the fight against mosquito-borne diseases. The first vaccine for malaria, developed by GSK Plc and its partners, was endorsed by the World Health Organization last year, enabling purchases from governments and international organizations. Another shot from the University of Oxford has shown that its protection can hold up a year after a booster. And Takeda says it's planning midstage trials of a vaccine against Zika.
Sanofi began work on its vaccine in 1997 and won Mexican approval for what it called Dengvaxia in 2015. The vaccine was soon introduced in 19 countries, but an analysis of clinical data in 2017 showed that for people receiving Dengvaxia who had never had dengue, the drug increased their risk of developing severe disease if they later became infected.
Takeda's path to Qdenga began in 2013 when it bought Inviragen Inc., a company in Colorado that had been working on the vaccine. Proving its efficacy and safety was complicated because dengue can be caused by four distinctive strains, and protection must be built against all of them. In some cases, patients who are reinfected with a different strain can suffer more severe symptoms because the neutralizing antibodies generated from the first infection may bind to the virus and increase its ability to enter cells. Although it's not clear, some researchers say a similar pattern may occur in vaccinated people—possibly the root cause of Dengvaxia's difficulties.
To ensure its vaccine didn't encounter similar issues, Takeda undertook more than four years of follow-up studies. The 1 million-plus pages of data it submitted to European authorities examined people who had been infected earlier as well as those who had never had dengue, something previous vaccines didn't do. "We learned from the failure," says Christophe Weber, Takeda's chief executive officer. "We designed the trial to specifically answer this question. That's what makes us confident."
Takeda's two-shot immunization is based on what's called the serotype 2 variant, with components of the three other strains attached. In a trial involving 1,800 young people in the Dominican Republic, Panama and the Philippines, the drug induced an immune response against all four strains that lasted at least four years after the injections.
That result allowed Takeda to proceed to a test involving more than 20,000 participants in Asia and Latin America. The vaccine cut hospitalizations of recipients by 84% compared with a placebo and prevented the illness in 61%, with no significant safety risks. Although the vaccine isn't equally effective against all four strains, Weber says, "what's important is to look at the overall efficacy."
Qdenga's strength is that it was built on a dengue virus, whereas Dengvaxia based its version on yellow fever, says Cameron Simmons, director of the Institute of Vector-Borne Disease at Monash University in Australia. "It's more dengue-like," he says. "So it's presenting more dengue virus antigens, if you like, to the immune system."
Duane Gubler, emeritus professor at the Duke-NUS Medical School in Singapore and a part of a team that worked on the vaccine at the US Centers for Disease Control and Prevention, cautions that Qdenga still risks setbacks similar to those that slowed Dengvaxia and recommends vigorous follow-up monitoring of recipients. "I don't think it's been really tested effectively against dengue three and four because we haven't had a lot of dengue three and four activity," Gubler says. "We have to wait for that."
After decades of delays, the first vaccine for dengue fever was introduced seven years ago. But that formula, from Sanofi, was soon found to be suitable only for people who had previously been infected with the disease, spurring researchers at Japan's Takeda Pharmaceutical Co. to redouble efforts on an alternative. That work is finally paying off, with their version expected to hit the market early next year.
Dengue infections have jumped eightfold over the past two decades, to almost 400 million a year, according to researchers at the University of Oxford. About half the world's population already lives in areas threatened by dengue, and scientists warn that climate change will likely hasten the spread of mosquitoes that carry the virus. Since 2013 the US has seen outbreaks in Florida, Hawaii and Texas. Europe saw local transmission in France and Croatia in 2010, and a 2012 outbreak on Portugal's Madeira island resulted in more than 2,000 cases.
Disclaimer: This article first appeared on Bloomberg, and is published by special syndication arrangement.



 Keep updated, follow The Business Standard's Google news channel
Keep updated, follow The Business Standard's Google news channel














